Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1


Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1
You know the right answer?
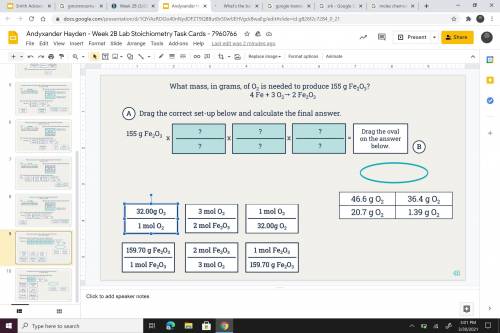
What mass, in grams of O2 is needed to produce 155 g Fe2 O3? 4 Fe + 3 O2---> 4 Fe + 3 o2 --> 2...
Questions



Social Studies, 05.10.2019 23:00

Chemistry, 05.10.2019 23:00

Mathematics, 05.10.2019 23:00


History, 05.10.2019 23:00


Chemistry, 05.10.2019 23:00

History, 05.10.2019 23:00





Mathematics, 05.10.2019 23:00

Biology, 05.10.2019 23:00

Mathematics, 05.10.2019 23:00


Biology, 05.10.2019 23:00