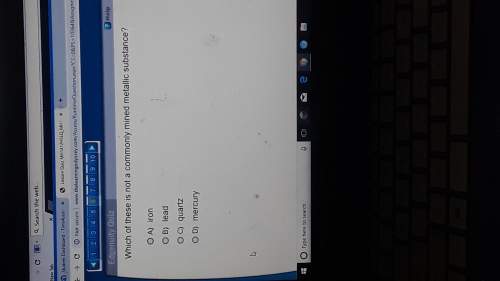
Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Calculate the Keq of the reaction CH4 + O2 ⇌ CO2 + 2H2O, given the following concentrations at equil...
Questions

Mathematics, 06.01.2021 01:00

English, 06.01.2021 01:00

English, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00

Business, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00




History, 06.01.2021 01:00


Social Studies, 06.01.2021 01:00


Mathematics, 06.01.2021 01:00

English, 06.01.2021 01:00

History, 06.01.2021 01:00

Mathematics, 06.01.2021 01:00