
Chemistry, 31.03.2021 20:50 alexkrol10
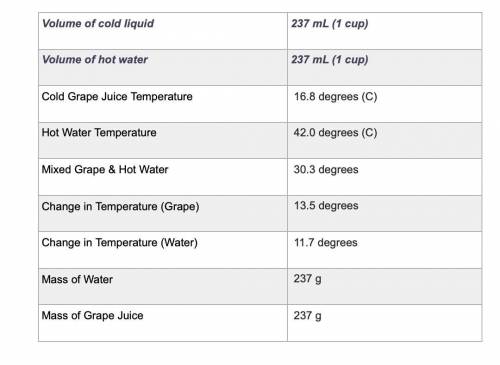
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the specific heat for the liquid you selected. Use the equation qwater = m × c × ΔT to calculate the heat lost by the hot water. Show your work using the problem-solving method shown in previous rubrics.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the speci...
Questions

Physics, 08.10.2019 11:10

Computers and Technology, 08.10.2019 11:10

Mathematics, 08.10.2019 11:10

Mathematics, 08.10.2019 11:10


Mathematics, 08.10.2019 11:10


Health, 08.10.2019 11:10


Chemistry, 08.10.2019 11:10




Social Studies, 08.10.2019 11:10




Geography, 08.10.2019 11:10


Mathematics, 08.10.2019 11:10



