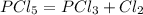I need help with the question below:
Where PCl5 and PCl3 are gasses
In the above e...


Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Questions





Biology, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10


Mathematics, 14.04.2021 17:10


Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10

Mathematics, 14.04.2021 17:10