
Chemistry, 01.04.2021 06:40 Knownothing
Calculate the pH of a solution formed by mixing 550.0 mL of 0.30 M HClO with 275.0 mL of 0.20 M CsClO. The Ka for HClO is 2.9 × 10-8.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1



Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Calculate the pH of a solution formed by mixing 550.0 mL of 0.30 M HClO with 275.0 mL of 0.20 M CsCl...
Questions

Biology, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01



Mathematics, 20.10.2020 04:01


English, 20.10.2020 04:01

Biology, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Spanish, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

English, 20.10.2020 04:01

Social Studies, 20.10.2020 04:01


Chemistry, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

![\frac{[ClO^-]}{[HClO]}](/tpl/images/1234/3126/d9b8f.png)
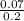 = 7.08
= 7.08

