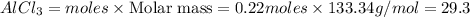PLEASE HELP
25.0g of Aluminum metal is combined with
45.Og Copper(ll) Chloride to produce Aluminum Chloride
and Copper metal
1. Write and balance the chemical equation.
2. Write the needed mole ratio between reactants.
3. What is the mole ratio you have from the data?
4. What is in excess and what is limited? (Show Work)
5. What is the theoretical yield of Aluminum Chloride?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
PLEASE HELP
25.0g of Aluminum metal is combined with
45.Og Copper(ll) Chloride to produce Alu...
45.Og Copper(ll) Chloride to produce Alu...
Questions

English, 02.07.2019 09:50



English, 02.07.2019 09:50

History, 02.07.2019 09:50

Social Studies, 02.07.2019 09:50




History, 02.07.2019 09:50


Social Studies, 02.07.2019 09:50

History, 02.07.2019 09:50



History, 02.07.2019 09:50

English, 02.07.2019 09:50

Mathematics, 02.07.2019 09:50


Geography, 02.07.2019 09:50

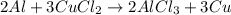
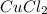 : 2 moles of
: 2 moles of 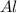
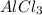 is 29.3 g
is 29.3 g 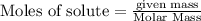
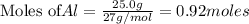
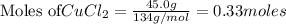
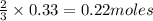 of
of