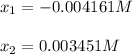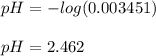Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
Calculate the pH during the titration of 20.00 mL of 0.1000 M HNO2(aq) with 0.1000 M KOH(aq) after 1...
Questions

French, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50


Mathematics, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50

Social Studies, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50

Geography, 08.03.2021 03:50



Biology, 08.03.2021 03:50

English, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50

Social Studies, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50

Mathematics, 08.03.2021 03:50



Spanish, 08.03.2021 03:50


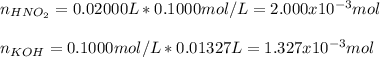
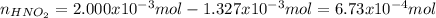
![[HNO_2]=\frac{6.73x10^{-4}mol}{0.01327L+0.02000L} =0.02023M](/tpl/images/1235/6198/ec867.png)
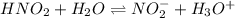
![Ka=\frac{[NO_2^-][H_3O^+]}{[HNO_2]}\\\\7.1x10^{-4}=\frac{x^2}{0.02023M-x}](/tpl/images/1235/6198/fbd05.png)