
Chemistry, 01.04.2021 21:40 kaywendel2008
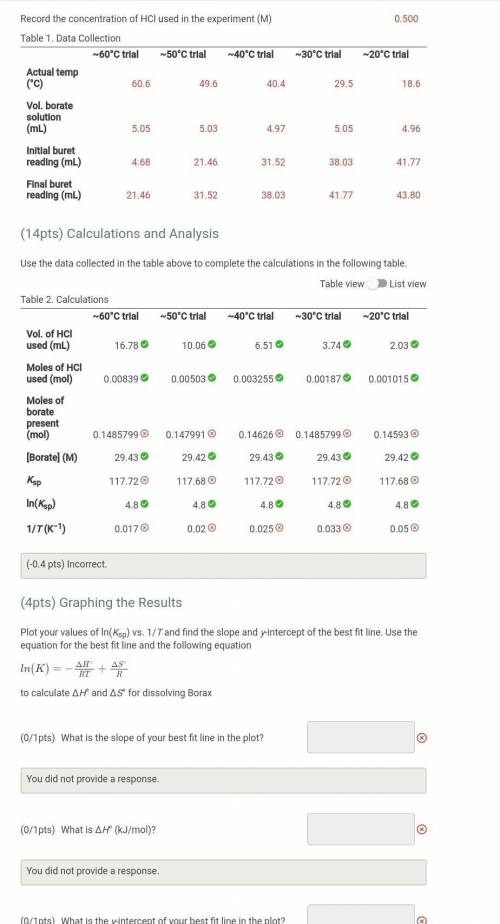
Plot your values of ln(Ksp) vs. 1/T and find the slope and y-intercept of the best fit line. Use the equation for the best fit line and the following equation
to calculate ΔH° and ΔS° for dissolving Borax
(0/1pts)
What is the slope of your best fit line in the plot?
at is ΔH° (kJ/mol)?
highlight_off
(0/1pts)
What is the y-intercept of your best fit line in the plot?
highlight_off
(-1 pts)
(0/1pts)
What is ΔS° (J/mol)?
l

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2
You know the right answer?
Plot your values of ln(Ksp) vs. 1/T and find the slope and y-intercept of the best fit line. Use the...
Questions



English, 15.01.2020 12:31


Social Studies, 15.01.2020 12:31

Mathematics, 15.01.2020 12:31

History, 15.01.2020 12:31

Biology, 15.01.2020 12:31



Mathematics, 15.01.2020 12:31



Mathematics, 15.01.2020 12:31



English, 15.01.2020 12:31






