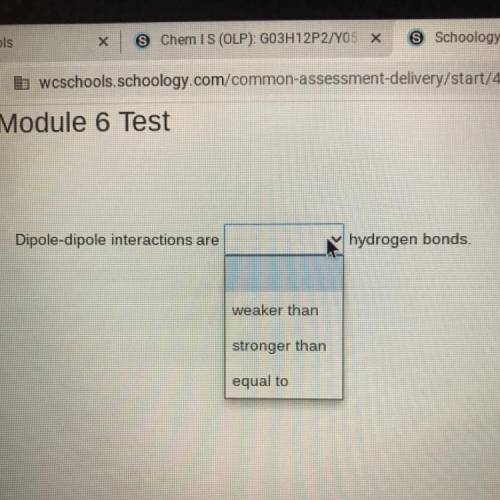
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.
...

Chemistry, 02.04.2021 01:00 postorivofarms
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2
You know the right answer?
Questions


Social Studies, 22.06.2019 07:30

Mathematics, 22.06.2019 07:30

Mathematics, 22.06.2019 07:30



Geography, 22.06.2019 07:30

History, 22.06.2019 07:30


English, 22.06.2019 07:30






History, 22.06.2019 07:30






