Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ...

Chemistry, 02.04.2021 01:40 adelawilliams60
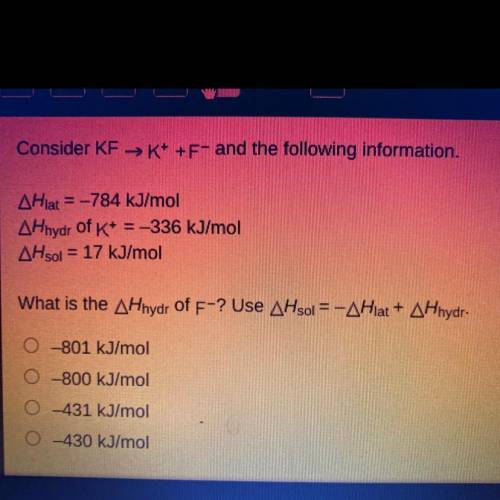
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ/mol
AHsol = 17 kJ/mol
What is the AHhydr of F-? Use AHsol = -AHlat + AHhydr.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2


Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Questions


History, 05.10.2020 01:01

English, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01


History, 05.10.2020 01:01


English, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01


History, 05.10.2020 01:01




Mathematics, 05.10.2020 01:01

Mathematics, 05.10.2020 01:01



History, 05.10.2020 01:01



