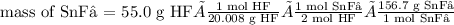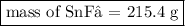Chemistry, 02.04.2021 17:40 aliceotter2007
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is prepared according
to the following equation:
Sn(s) + 2HF(aq) → SnF2(aq) + H2(g)
How many grams of tin(II) fluoride can be produced
from 55.0 g of hydrogen fluoride if there is plenty of tin
available to react?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is pre...
Questions

Computers and Technology, 08.03.2021 22:30



Computers and Technology, 08.03.2021 22:30


Mathematics, 08.03.2021 22:30


Biology, 08.03.2021 22:30



English, 08.03.2021 22:30



English, 08.03.2021 22:30

Mathematics, 08.03.2021 22:30

Chemistry, 08.03.2021 22:30

SAT, 08.03.2021 22:30



Biology, 08.03.2021 22:30