

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
You are given a stock solution of 500.0 mL of 1.00M magnesium chloride solution. Calculate the volum...
Questions

Mathematics, 09.06.2020 23:57



Mathematics, 09.06.2020 23:57


Mathematics, 09.06.2020 23:57

History, 09.06.2020 23:57



Social Studies, 09.06.2020 23:57


Social Studies, 09.06.2020 23:57

Mathematics, 09.06.2020 23:57



Mathematics, 09.06.2020 23:57



Mathematics, 09.06.2020 23:57

Computers and Technology, 09.06.2020 23:57

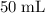 of the stock solution would be required.
of the stock solution would be required. 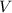 contains a solute with a concentration of
contains a solute with a concentration of  . The quantity
. The quantity 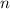 of that solute in this solution would be:
of that solute in this solution would be: 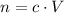 .
.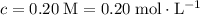 . The volume of this solution is
. The volume of this solution is 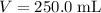 . Calculate the quantity of the solute (magnesium chloride) in the required solution:
. Calculate the quantity of the solute (magnesium chloride) in the required solution: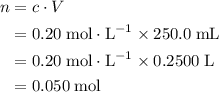 .
.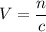 .
.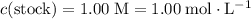 .
.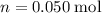 .
.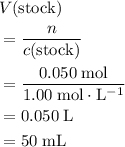 .
.

