
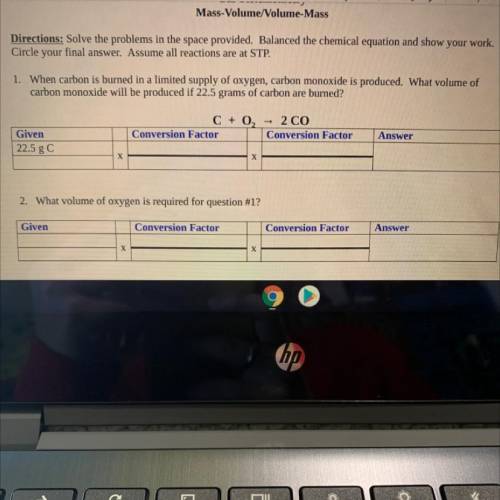
Chemistry, 02.04.2021 22:00 berlyntyler
Mass-Volume/Volume-Mass
Directions: Solve the problems in the space provided. Balanced the chemical equation and show your work.
Circle your final answer. Assume all reactions are at STP.
1. When carbon is burned in a limited supply of oxygen, carbon monoxide is produced. What volume of
carbon monoxide will be
produced if 22.5 grams of carbon are burned?
C + O2 = 2 CO

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Mass-Volume/Volume-Mass
Directions: Solve the problems in the space provided. Balanced the chemica...
Questions

Mathematics, 04.02.2020 02:50



Biology, 04.02.2020 02:50

Mathematics, 04.02.2020 02:50

Chemistry, 04.02.2020 02:50

History, 04.02.2020 02:50


Mathematics, 04.02.2020 02:50



Mathematics, 04.02.2020 02:50

Social Studies, 04.02.2020 02:50

Advanced Placement (AP), 04.02.2020 02:50

Mathematics, 04.02.2020 02:50

Health, 04.02.2020 02:50

Social Studies, 04.02.2020 02:50

Mathematics, 04.02.2020 02:50

Mathematics, 04.02.2020 02:50

History, 04.02.2020 02:50



