Ammonia is produced by the chemical reaction of hydrogen and nitrogen
N2 + 2H2 → 2NH3
H...

Chemistry, 03.04.2021 02:40 thegreentnt5025
Ammonia is produced by the chemical reaction of hydrogen and nitrogen
N2 + 2H2 → 2NH3
How many moles of H2 are needed to react with 1.8 moles of N2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:50
Your roll: experienced electron speech is adressed to: a new "freshman class" of electrons job: write a speech task: you are to pretend that you are giving a speech to a new group of electrons. be sure to mention their placement in an atom, their charge, and their role in chemical bonding (ionic and covalent) be specific!
Answers: 3

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2
You know the right answer?
Questions

History, 06.02.2022 01:00



Geography, 06.02.2022 01:00

Mathematics, 06.02.2022 01:00

Mathematics, 06.02.2022 01:00

Mathematics, 06.02.2022 01:00

Mathematics, 06.02.2022 01:00


History, 06.02.2022 01:00



English, 06.02.2022 01:00

Mathematics, 06.02.2022 01:00


Social Studies, 06.02.2022 01:00

Biology, 06.02.2022 01:00

Social Studies, 06.02.2022 01:00

Physics, 06.02.2022 01:00

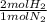 = 3.6 mol H₂
= 3.6 mol H₂

