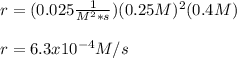Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is...

Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is 0.025, and the reaction is second order in Xe and first order in F2.
What is the rate of the reaction if [Xe] is 0.25 M, and [F2] is 0.4 M?
4.0 × 10–^4
2.5 × 10–^3
1.5 × 10–^3
6.3 × 10–^4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1


Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
Questions

Mathematics, 27.08.2021 02:30




History, 27.08.2021 02:30




Mathematics, 27.08.2021 02:30

Mathematics, 27.08.2021 02:30



Mathematics, 27.08.2021 02:30

Mathematics, 27.08.2021 02:30

Mathematics, 27.08.2021 02:30




English, 27.08.2021 02:30

![r=k[Xe]^2[F_2]](/tpl/images/1238/0471/c5483.png)