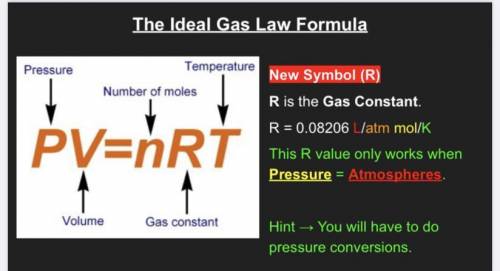
Chemistry, 03.04.2021 14:30 msitez5993
calculate the volume in L occupied by 13.2 moles of carbon dioxide gas if the pressure is 250 kPa at 73.6 degrees Celsius

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
calculate the volume in L occupied by 13.2 moles of carbon dioxide gas if the pressure is 250 kPa at...
Questions

Mathematics, 16.12.2020 16:10






Mathematics, 16.12.2020 16:10


Mathematics, 16.12.2020 16:10



Mathematics, 16.12.2020 16:10


Business, 16.12.2020 16:10




History, 16.12.2020 16:10