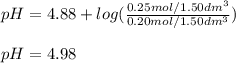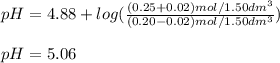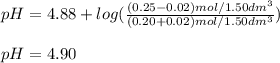A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate (CH3CH2COONa) in 1.50 dm3.
What is the pH of this buffer?
Enter your answer using two decimal places.
What is the pH of the buffer after the addition of 0.02 mol of NaOH?
What is the pH of the buffer after the addition of 0.02 mol of HI?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2


Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate...
Questions




















![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/1238/6169/33848.png)