Chemistry, 05.04.2021 14:00 reginaldlegette
A beaker contains 1.0 M NaOH(aq) solution. To produce a NaOH(aq) solution of molar concentration 0.20 M, we transfer 2.0 ml (cm^3) out of this beaker into a 25 ml cylinder and add distilled water until the total volume reading becomes
A) 12ml
B)10ml
C)8ml
D)5ml
E)2ml

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
A beaker contains 1.0 M NaOH(aq) solution. To produce a NaOH(aq) solution of molar concentration 0.2...
Questions



Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01







Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01



Mathematics, 23.08.2020 01:01

Social Studies, 23.08.2020 01:01



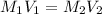
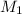 = molarity of stock
= molarity of stock 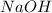 solution = 1.0 M
solution = 1.0 M
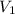 = volume of stock
= volume of stock