
* look at the photo *
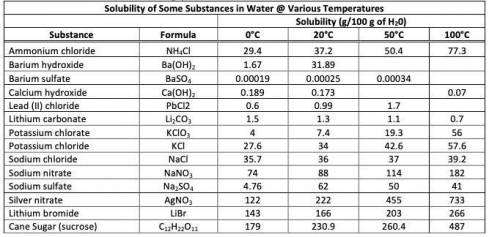
1. Saturated solutions of each of the following compounds are made at 20°C. Circle the letter(s) of the solution(s), which will form a precipitate upon heating.
a) NaCl b) Na2SO4 c) Li2CO3 d) Sucrose
2. A saturated solution of potassium chloride is prepared in 100.0 g of water at 20°C. If the solution is heated to
50°C, how much more KCl must be added to obtain a saturated solution?
3. A saturated solution of sucrose in 1000.0 g of boiling water is cooled to 20°C. What mass of rock candy will be formed?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
* look at the photo *
1. Saturated solutions of each of the following compounds are made at 20°C. C...
Questions


Mathematics, 29.01.2021 17:00


Mathematics, 29.01.2021 17:00



Law, 29.01.2021 17:00

Social Studies, 29.01.2021 17:00

Business, 29.01.2021 17:00

English, 29.01.2021 17:00

History, 29.01.2021 17:00




English, 29.01.2021 17:00


History, 29.01.2021 17:00


Geography, 29.01.2021 17:00

Mathematics, 29.01.2021 17:00



