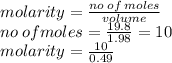Given this image, please try to decipher what the question is asking and work it out.
Given:
...

Chemistry, 06.04.2021 20:20 labrandonanderson00
Given this image, please try to decipher what the question is asking and work it out.
Given:
V = .49 L
m = 1.98 g/per packet
total mass = 19.8 g
I think that I am supposed to solve for molarity, but I don't think that there is enough information given. Thanks is advance & no fake answers please.


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Questions



History, 24.07.2019 05:10


Mathematics, 24.07.2019 05:10



Chemistry, 24.07.2019 05:10


Mathematics, 24.07.2019 05:10


Physics, 24.07.2019 05:10

Mathematics, 24.07.2019 05:10


English, 24.07.2019 05:10



Biology, 24.07.2019 05:10

Computers and Technology, 24.07.2019 05:10

Social Studies, 24.07.2019 05:10