

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions

Mathematics, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10

Social Studies, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10

Chemistry, 26.02.2021 22:10

Chemistry, 26.02.2021 22:10

Social Studies, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10

Mathematics, 26.02.2021 22:10


Mathematics, 26.02.2021 22:10

History, 26.02.2021 22:10




Chemistry, 26.02.2021 22:10


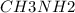 ) with 0.493 M
) with 0.493 M 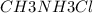 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?

