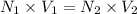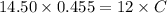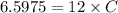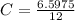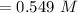Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
It took 14.50 mL of 0.455M NaOH to fully neutralize 12.0mL of HCl. What is the concentration of the...
Questions

Mathematics, 04.03.2021 19:00


Mathematics, 04.03.2021 19:00


Mathematics, 04.03.2021 19:00


Mathematics, 04.03.2021 19:00






Mathematics, 04.03.2021 19:00


Mathematics, 04.03.2021 19:00

Health, 04.03.2021 19:00

Mathematics, 04.03.2021 19:00


English, 04.03.2021 19:00

Social Studies, 04.03.2021 19:00