

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
34 g of O2 are reacted with excess Cs, causing a production of 199 g of Cs2O. What is the percent yi...
Questions





Mathematics, 04.06.2021 21:00



Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00


Mathematics, 04.06.2021 21:00



Mathematics, 04.06.2021 21:00



Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00

Mathematics, 04.06.2021 21:00

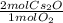 = 2.125 mol Cs₂O
= 2.125 mol Cs₂O

