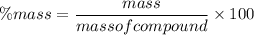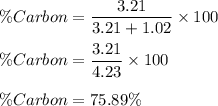Chemistry, 07.04.2021 06:30 xxtonixwilsonxx
A compound is found to be made up of 3.21 g Carbon and 1.02 g Oxygen. Determine the percent composition from this data?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

You know the right answer?
A compound is found to be made up of 3.21 g Carbon and 1.02 g Oxygen. Determine the percent composit...
Questions


English, 13.12.2021 03:20

Mathematics, 13.12.2021 03:20






Mathematics, 13.12.2021 03:30



Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30

Mathematics, 13.12.2021 03:30

Spanish, 13.12.2021 03:30

World Languages, 13.12.2021 03:30

History, 13.12.2021 03:30



Mathematics, 13.12.2021 03:30