
Chemistry, 07.04.2021 19:50 nyasiasaunders1234
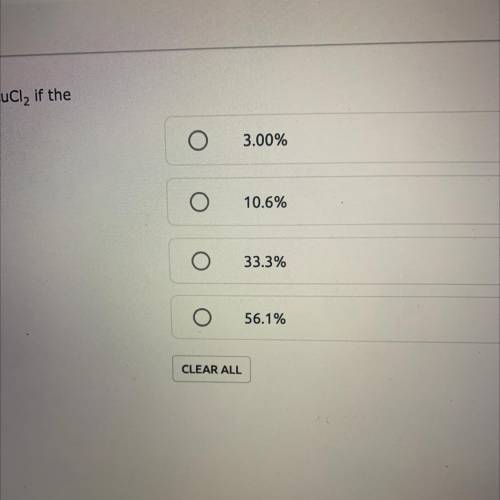
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield is 18.81g? % Yield = (Actual Yield/Theoretical Yield) x 100

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield...
Questions

English, 06.12.2021 19:10

English, 06.12.2021 19:10

Computers and Technology, 06.12.2021 19:10




Computers and Technology, 06.12.2021 19:10


Mathematics, 06.12.2021 19:10








Mathematics, 06.12.2021 19:20

Mathematics, 06.12.2021 19:20


English, 06.12.2021 19:20



