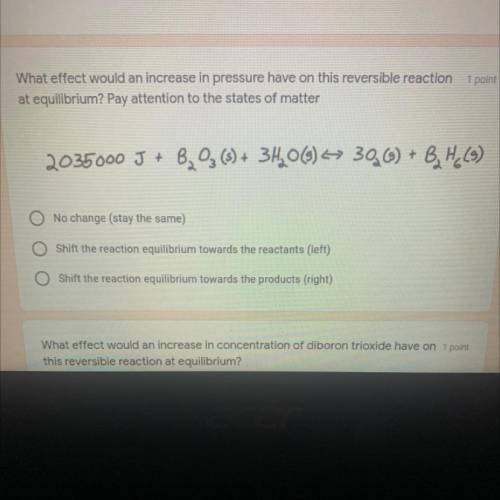
What effect would an increase in pressure have on this reversible reaction at Equilibrium
...

Chemistry, 07.04.2021 20:20 chessacs6540
What effect would an increase in pressure have on this reversible reaction at Equilibrium

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Questions

Mathematics, 12.12.2021 20:10

Mathematics, 12.12.2021 20:10



Mathematics, 12.12.2021 20:10




Biology, 12.12.2021 20:10


English, 12.12.2021 20:10

Mathematics, 12.12.2021 20:10




Mathematics, 12.12.2021 20:10

Social Studies, 12.12.2021 20:10

Mathematics, 12.12.2021 20:10

Mathematics, 12.12.2021 20:10




