
Chemistry, 08.04.2021 01:20 emmaja121003
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
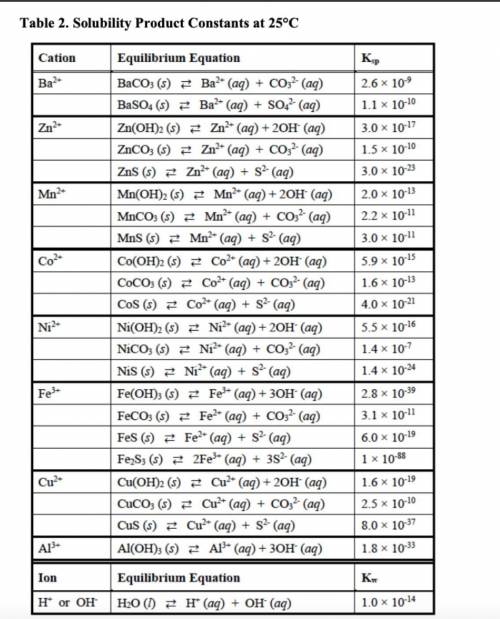
out the corresponding solubility equilibrium equations that would give rise to a precipitate with ammonia. Be careful to use the appropriate arrows (⇄ vs. →), depending on the compound’s solubility. Using the Ksp values provided in Table 2, predict whether it would form using 5 drops (0.25 mL) of 3 M NH3 and 1 mL of 0.10 M metal nitrate solution.
Your answer must include:
• The relevant chemical equation(s) showing dissociation or equilibrium of the
products into their ionic components.
• The Ksp values and equations
• Calculations showing how concentrations were determined
• Unique Qsp calculations
• A comparison of Qsp and Ksp, and discussion of what this comparison predicts
about precipitate (solid) formation.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

You know the right answer?
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
out the corresp...
Questions


Mathematics, 02.09.2020 04:01

Chemistry, 02.09.2020 04:01











History, 02.09.2020 04:01



History, 02.09.2020 04:01



Mathematics, 02.09.2020 04:01




