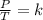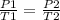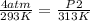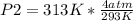Chemistry, 08.04.2021 09:10 liptontea4
You had a closed tank of air at a pressure of 4 atm and temperature of 20 degrees Celsius. When the tank and the air are heated to 40 degrees Celsius, what is the pressure if the volume remains constant?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
You had a closed tank of air at a pressure of 4 atm and temperature of 20 degrees Celsius. When the...
Questions


Computers and Technology, 24.07.2019 07:30

Mathematics, 24.07.2019 07:30


Mathematics, 24.07.2019 07:30

Chemistry, 24.07.2019 07:30

Mathematics, 24.07.2019 07:30

Mathematics, 24.07.2019 07:30

Biology, 24.07.2019 07:30

History, 24.07.2019 07:30

History, 24.07.2019 07:30





Geography, 24.07.2019 07:30


Biology, 24.07.2019 07:30

English, 24.07.2019 07:30