

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

You know the right answer?
A container at equilibrium contains the following concentrations: [Hz] - 0.30 M and [HI]
0.90 M. Th...
Questions

Mathematics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00


Social Studies, 01.12.2020 01:00




Mathematics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

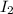 at equilibrium is 0.45 M
at equilibrium is 0.45 M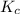
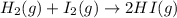
![K_c=\frac{[HI]^2}{[H_2]^1[I_2]^1}](/tpl/images/1245/8126/da9c3.png)
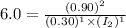
![[I_2]=0.45M](/tpl/images/1245/8126/936c9.png)


