
Chemistry, 08.04.2021 18:40 silviamgarcia
A reaction is first order with respect to reactant A and second order with respect to reactant B. Starting with [A] = 0.175 M and [B] = 0.00250 M, the reaction rate is 2.83 x 10−5 M. S−1. What is the rate constant k? Show your work.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
A reaction is first order with respect to reactant A and second order with respect to reactant B. St...
Questions

History, 09.06.2021 14:00

Spanish, 09.06.2021 14:00

English, 09.06.2021 14:00

History, 09.06.2021 14:00

History, 09.06.2021 14:00

Social Studies, 09.06.2021 14:00


Physics, 09.06.2021 14:00

Chemistry, 09.06.2021 14:00

Biology, 09.06.2021 14:00

Mathematics, 09.06.2021 14:00

Mathematics, 09.06.2021 14:00

English, 09.06.2021 14:00

Chemistry, 09.06.2021 14:00


Physics, 09.06.2021 14:00


English, 09.06.2021 14:00


Physics, 09.06.2021 14:00

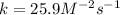
![r=k[A][B]^2](/tpl/images/1246/4928/487f0.png)
![k=\frac{r}{[A][B]^2} \\\\k=\frac{2.83x10^{-5}M/s}{(0.175M)(0.00250M)^2}\\\\k=25.9M^{-2}s^{-1}](/tpl/images/1246/4928/29bac.png)


