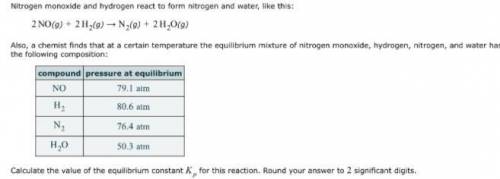
Nitrogen monoxide and hydrogen react to form nitrogen and water, like this: 2NO(g) 2H2(g) N2(g) 2H2O(g)Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen monoxide, hydrogen, nitrogen, and water has the following composition:compoundpressure at equilibriumCalculate the value of the equilibrium constant Kp for this reaction. Round your answer to significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Nitrogen monoxide and hydrogen react to form nitrogen and water, like this: 2NO(g) 2H2(g) N2(g) 2H2O...
Questions



Mathematics, 14.11.2019 20:31









Mathematics, 14.11.2019 20:31


Social Studies, 14.11.2019 20:31






Mathematics, 14.11.2019 20:31