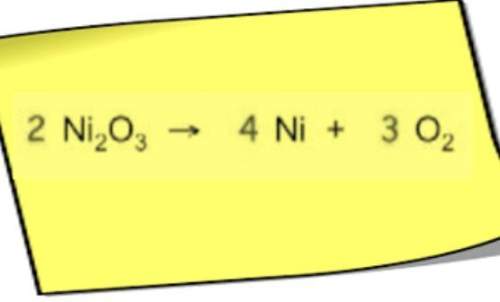
Chemistry, 08.04.2021 21:10 thedeathlord123
If 50.0 ml from the above solution is evaporated. What will be the new molarity? Show Your Work

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
If 50.0 ml from the above solution is evaporated. What will be the new molarity? Show Your Work...
Questions

Health, 27.02.2021 01:40

Mathematics, 27.02.2021 01:40


English, 27.02.2021 01:40


Computers and Technology, 27.02.2021 01:40

English, 27.02.2021 01:40


History, 27.02.2021 01:40


Mathematics, 27.02.2021 01:40

Mathematics, 27.02.2021 01:40

Health, 27.02.2021 01:40





Chemistry, 27.02.2021 01:40

Mathematics, 27.02.2021 01:40