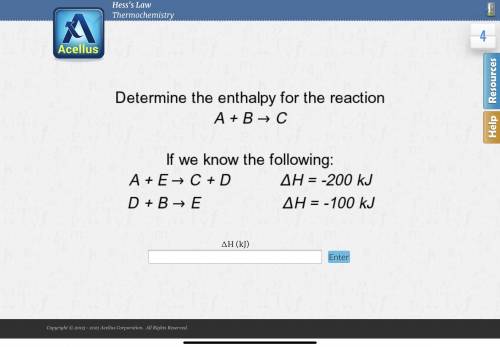
Determine the enthalpy for the reaction A + B -> C
If we know the following:
A + E ->...

Chemistry, 08.04.2021 22:20 Lilbre6999
Determine the enthalpy for the reaction A + B -> C
If we know the following:
A + E -> C + D deltaH = -200 kJ
D + B -> E deltaH = -100 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Questions


Mathematics, 08.11.2019 04:31

Physics, 08.11.2019 04:31

History, 08.11.2019 04:31

History, 08.11.2019 04:31


Mathematics, 08.11.2019 04:31

History, 08.11.2019 04:31

Computers and Technology, 08.11.2019 04:31





Computers and Technology, 08.11.2019 04:31

Computers and Technology, 08.11.2019 04:31








