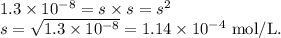Chemistry, 09.04.2021 01:00 hargurgill20
PbSO4 has a Ksp = 1.3 * 10-8 (mol/L)2. I will mark brainliest if you answer all three questions. Thank you so so so much!!!


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
PbSO4 has a Ksp = 1.3 * 10-8 (mol/L)2.
I will mark brainliest if you answer all three questions. Th...
Questions


Social Studies, 30.08.2019 09:50


English, 30.08.2019 09:50

Mathematics, 30.08.2019 09:50


Mathematics, 30.08.2019 09:50


Physics, 30.08.2019 09:50





Biology, 30.08.2019 09:50

Mathematics, 30.08.2019 09:50

History, 30.08.2019 09:50



History, 30.08.2019 09:50

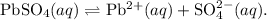
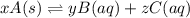 ,
,![K_{sp} = [B]^y [C]^z](/tpl/images/1247/6109/8bc3e.png) .
.![K_{sp} = \mathrm{[Pb^{2+}] [SO_4^{2-}]}](/tpl/images/1247/6109/6acd9.png) .
.