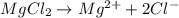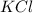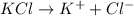The following solutions are prepared by dissolving the requisite amount of solute in water to obtain the desired concentrations. Rank the solutions according to their respective osmotic pressures in decreasing order assuming the complete dissociation of ionic compounds. Rank from highest to lowest osmotic pressure. To rank items as equivalent, overlap them.
A. 1 M MgCl2
B. 1 M KCI
C. 1 M C12
D. H22011
1. Highest osmotic pressure
2. Lowest osmotic pressure

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
The following solutions are prepared by dissolving the requisite amount of solute in water to obtain...
Questions

Geography, 06.10.2019 20:10

Mathematics, 06.10.2019 20:10

Mathematics, 06.10.2019 20:10


English, 06.10.2019 20:10

Mathematics, 06.10.2019 20:10

Spanish, 06.10.2019 20:10

Mathematics, 06.10.2019 20:10




Mathematics, 06.10.2019 20:10



Mathematics, 06.10.2019 20:10

Computers and Technology, 06.10.2019 20:10

Health, 06.10.2019 20:10


Biology, 06.10.2019 20:10

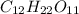

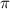 = osmotic pressure
= osmotic pressure