
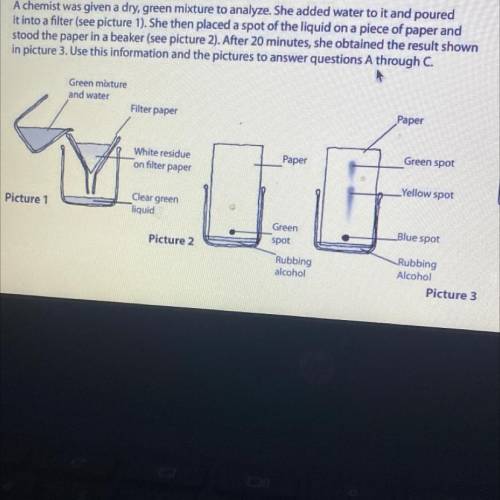
A chemist was given a dry, green mixture to analyze. She added water to it and poured
it into a filter (see picture 1). She then placed a spot of the liquid on a piece of paper and
stood the paper in a beaker (see picture 2). After 20 minutes, she obtained the result show
in picture 3. Use this information and the pictures to answer questions A through C.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3


Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
A chemist was given a dry, green mixture to analyze. She added water to it and poured
it into a fil...
Questions



Mathematics, 10.03.2020 09:08



Computers and Technology, 10.03.2020 09:08











Computers and Technology, 10.03.2020 09:08





