
Chemistry, 10.04.2021 01:10 mendezmarco2004
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds as completely as possible. Which TWO of the following statements are correct? *
A. CO(g) is the limiting reactant.
B. O2(g) is the limiting reactant.
C. 4mol of CO(g) reacts.
D. 2mol of CO(g) remains unreacted.
E. 144g of CO2(g) is produced.
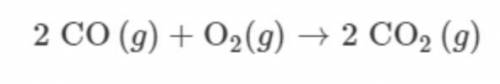

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1


Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
A 112g sample of CO(g) is combined with a 32g sample of O2(g) and the reaction represented proceeds...
Questions

Mathematics, 02.12.2021 23:00


Chemistry, 02.12.2021 23:00


Mathematics, 02.12.2021 23:00


Mathematics, 02.12.2021 23:00

Mathematics, 02.12.2021 23:00




English, 02.12.2021 23:00







English, 02.12.2021 23:00

Mathematics, 02.12.2021 23:00



