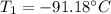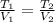Chemistry, 10.04.2021 03:50 ehgdhjahag
An ideal gas in a sealed container has an initial volume of 2.80 L. At constant pressure, it is cooled to 18.00 °C, where its
final volume is 1.75 L. What was the initial temperature?
Ti =
'c

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
An ideal gas in a sealed container has an initial volume of 2.80 L. At constant pressure, it is cool...
Questions


Mathematics, 19.08.2019 04:30


English, 19.08.2019 04:30


English, 19.08.2019 04:30




Mathematics, 19.08.2019 04:30



Mathematics, 19.08.2019 04:30

Mathematics, 19.08.2019 04:30


Mathematics, 19.08.2019 04:30

English, 19.08.2019 04:30



Mathematics, 19.08.2019 04:30