Question 1:
How many atoms are in 25.00 g of Li.
A. 2.393 x 10^24 atoms of Li
B. 1.333...

Chemistry, 10.04.2021 08:40 jalenshayewilliams
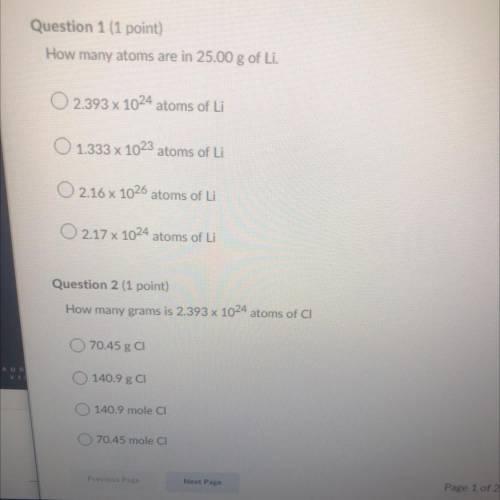
Question 1:
How many atoms are in 25.00 g of Li.
A. 2.393 x 10^24 atoms of Li
B. 1.333 x 10^23 atoms of Li
C. 2.16 x 10^26 atoms of Li
D. 2.17 x 10^24 atoms of Li
Question 2:
How many grams is 2.393 x 10^24 atoms of CI
A. 70.45 g CI
B. 140.9 g CI
C. 140.9 mole CI
D. 70.45 mole CI
Please try to answer both questions! Thanks for all the effort :)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Questions

Mathematics, 05.05.2020 14:19

Mathematics, 05.05.2020 14:19

History, 05.05.2020 14:19



Spanish, 05.05.2020 14:19


Health, 05.05.2020 14:19


Mathematics, 05.05.2020 14:19

Mathematics, 05.05.2020 14:19

Mathematics, 05.05.2020 14:19

History, 05.05.2020 14:19

Mathematics, 05.05.2020 14:19

Mathematics, 05.05.2020 14:19



History, 05.05.2020 14:19


Geography, 05.05.2020 14:19



