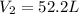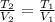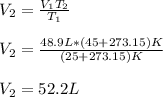Chemistry, 11.04.2021 05:00 madiforkner
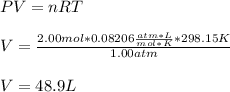
A gas system contains 2.00 moles of O2 and CO2 gas, has an initial temperature of 25.0 oC and is under 1.00 atm of pressure. If the pressure remains constant and the temperature is raised to 45.0 oC, then what will the new volume (assuming a closed system) be?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
A gas system contains 2.00 moles of O2 and CO2 gas, has an initial temperature of 25.0 oC and is und...
Questions



English, 05.10.2019 04:20



History, 05.10.2019 04:30









Mathematics, 05.10.2019 04:30

Computers and Technology, 05.10.2019 04:30


Biology, 05.10.2019 04:30

Mathematics, 05.10.2019 04:30