
Chemistry, 11.04.2021 08:20 amortegaa805
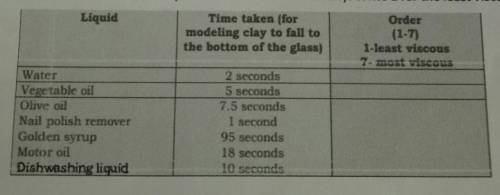
Direction: Using the chart above, number the liquids in order of their viscosity. Write 1 for the least viscous liquid and 7 forthe most viscous
Guide Questions:
1. Which liquid is the most viscous? How do you know?
2. Which liquid is the least viscous? How do you know?
3. Explain viscosity in your own words.
4. Compare how these liquids flow with how you think lava flows.
Why do some types of lava travel faster than others?
5. How does composition affect the viscosity of magma?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Follow the steps provided in the simulation to add water to the graduated cylinder, select one of the three samples (copper, silver, or gold), set its mass to the values given in the statements below, and calculate its density. here is a summary of the steps required: add water by clicking and holding prepare a known volume of water button. until the desired volume of water has been added. if more than the desired volume is added, click the reset button. button and redo the procedure. a single click will add about 21.0 ml of water. to set the mass, click and hold weigh out metal button. until the desired amount of metal is added to the weighing pan. once the desired mass of the metal is added, release the button. transfer the metal to water and then click on calculate density button. to see how the density is calculated using water displacement to measure the volume of the solid. to save time you can approximate the initial volume of water to â±1 ml and the initial mass of the solid to â±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? check all that apply.
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
Direction: Using the chart above, number the liquids in order of their viscosity. Write 1 for the le...
Questions


Physics, 08.01.2020 06:31

Advanced Placement (AP), 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31

History, 08.01.2020 06:31


Mathematics, 08.01.2020 06:31



Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31



Mathematics, 08.01.2020 06:31


Mathematics, 08.01.2020 06:31

Mathematics, 08.01.2020 06:31


Mathematics, 08.01.2020 06:31



