
Chemistry, 11.04.2021 08:40 susannaking5852
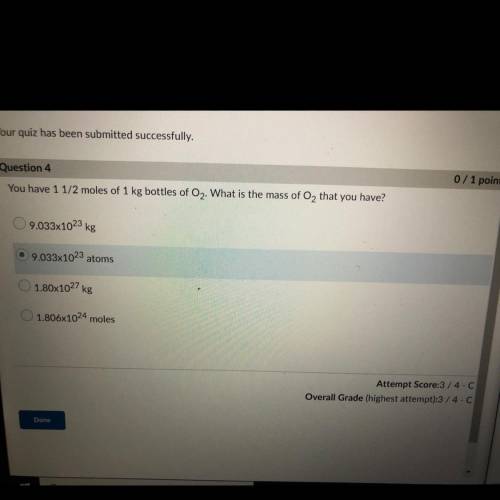
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg
B. 9.033x10^23 atoms
C. 1.80x10^27 kg
D. 1.806x10^24 moles
It is not B as I have already tried it :(

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg...
Questions

Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01


English, 18.10.2020 15:01



Biology, 18.10.2020 15:01

World Languages, 18.10.2020 15:01

Chemistry, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01


Mathematics, 18.10.2020 15:01




Social Studies, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Mathematics, 18.10.2020 15:01

Advanced Placement (AP), 18.10.2020 15:01

Health, 18.10.2020 15:01



