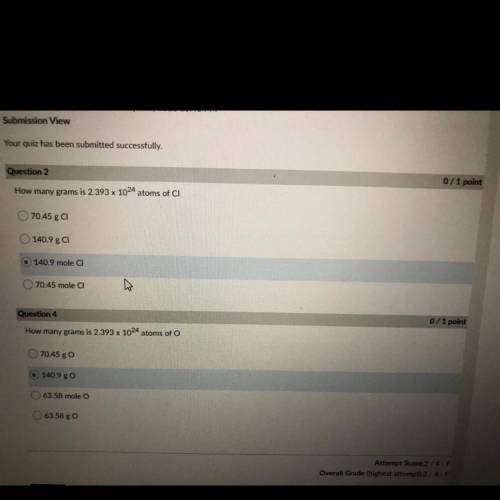
How many grams is 2.393 x 10^24 atoms of CI
70.45 g CI
140.9 ga
140.9 mole CI
70....


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

You know the right answer?
Questions



English, 12.09.2019 17:20

History, 12.09.2019 17:20


Mathematics, 12.09.2019 17:20








Chemistry, 12.09.2019 17:20




Mathematics, 12.09.2019 17:20


History, 12.09.2019 17:20