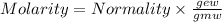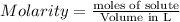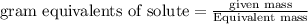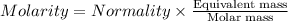Chemistry, 11.04.2021 14:00 joelpimentel
The relation between molarity and normality is expresses as: a) M = N x g. e.w/g. m.w
b) M = N x g. m.w/ g. e.w
c) M = N x no. of equiv./mole
d) M = N x no. of g. m.w/mole

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
The relation between molarity and normality is expresses as: a) M = N x g. e.w/g. m.w
b) M = N x g....
Questions









Mathematics, 10.07.2019 01:20

Mathematics, 10.07.2019 01:20





Computers and Technology, 10.07.2019 01:20




History, 10.07.2019 01:20