
Chemistry, 11.04.2021 23:30 sarahelisabeth444
Help please
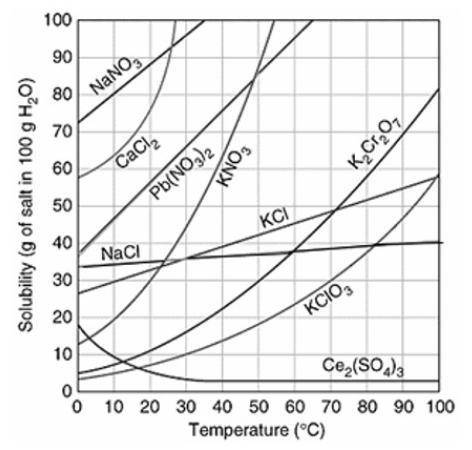
Assuming that the trends continue, which of the following compounds do you predict will have the GREATEST solubility at 120°C?
A.
Ce2(SO4)3
B.
K2Cr2O7
C.
Pb(NO3)2
D.
NaCl

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
Help please
Assuming that the trends continue, which of the following compounds do you predict will...
Questions


Mathematics, 04.02.2020 14:59

Arts, 04.02.2020 14:59





Mathematics, 04.02.2020 14:59

Social Studies, 04.02.2020 14:59

History, 04.02.2020 14:59

Advanced Placement (AP), 04.02.2020 14:59

Mathematics, 04.02.2020 14:59



Chemistry, 04.02.2020 14:59

Mathematics, 04.02.2020 14:59




Mathematics, 04.02.2020 14:59



