
Chemistry, 12.04.2021 08:20 shadoris26
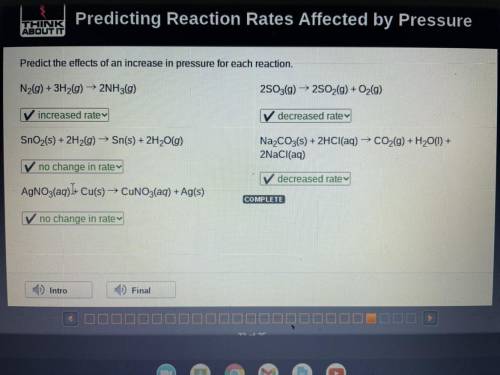
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g) ► 2S02(g) + O2(g)
SnO2(s) + 2H2(g) → Sn(s) + 2H20(g)
Na2CO3(s) + 2HCl(aq) → CO2(g) + H20(1) +
2NaCl(aq)
AgNO3(aq))+ Cu(s) → CUNO3(aq) + Ag(s)
COMPLETE

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g)...
Questions

Biology, 03.02.2020 13:51


Chemistry, 03.02.2020 13:51

Mathematics, 03.02.2020 13:51







English, 03.02.2020 13:51

Mathematics, 03.02.2020 13:51


Mathematics, 03.02.2020 13:51








