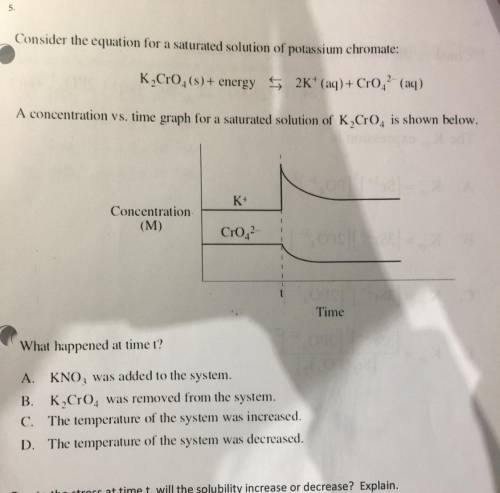
5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5...

Chemistry, 12.04.2021 14:00 Knownothing
5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5 2K+ (aq) + Cro. (aq)
A concentration vs. time graph for a saturated solution of K Cr0is shown below.
K+
Concentration
(M)
Cr02
Time
What happened at time t?
A. KNO, was added to the system.
B. K Cro. was removed from the system.
C. The temperature of the system was increased.
D. The temperature of the system was decreased.
Due to the stress at time t, will the solubility increase or decrease? Explain.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2


Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Questions









Mathematics, 15.04.2020 05:13



Computers and Technology, 15.04.2020 05:13








Computers and Technology, 15.04.2020 05:13



