
Chemistry, 12.04.2021 18:10 jameskarbar9p8c9d2
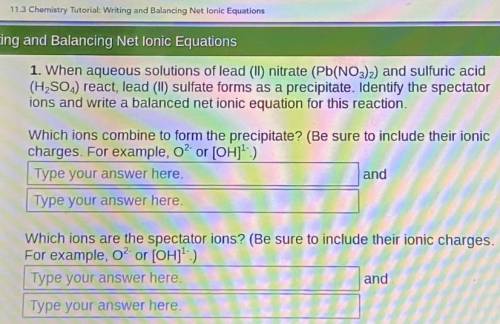
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II)...
Questions


Computers and Technology, 12.07.2021 20:40


Mathematics, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40


History, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40


Mathematics, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40

Engineering, 12.07.2021 20:40


English, 12.07.2021 20:40

Mathematics, 12.07.2021 20:40




Mathematics, 12.07.2021 20:40

English, 12.07.2021 20:40



