
Chemistry, 12.04.2021 18:10 kenisonpaigebosma
Fill in the coefficients to complete the balanced net ionic equation. (Use 1 to indicate when a coefficient is not needed.)
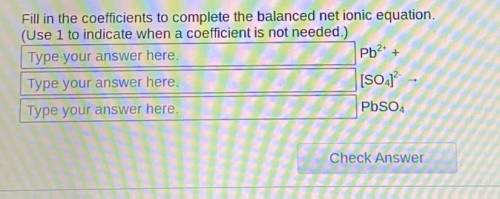

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Fill in the coefficients to complete the balanced net ionic equation. (Use 1 to indicate when a coef...
Questions


History, 28.08.2019 01:30

History, 28.08.2019 01:30

English, 28.08.2019 01:30


Mathematics, 28.08.2019 01:30



Mathematics, 28.08.2019 01:30


Mathematics, 28.08.2019 01:30

English, 28.08.2019 01:30

Biology, 28.08.2019 01:30



Computers and Technology, 28.08.2019 01:30



World Languages, 28.08.2019 01:30



