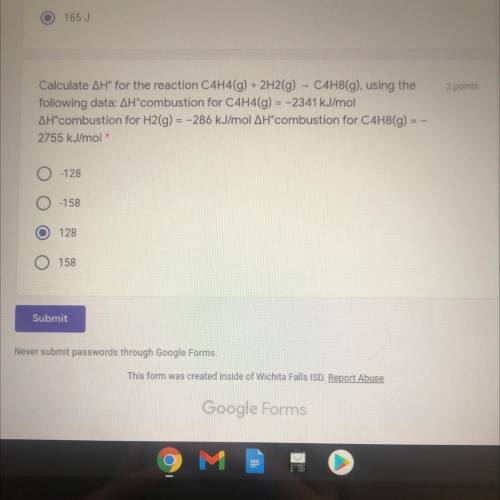
ASAP PLS
Calculate AH® for the reaction C4H4(g) + 2H2(g) - C4H8(g), using the
following data:...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

You know the right answer?
Questions

World Languages, 04.09.2021 21:10


Mathematics, 04.09.2021 21:10




English, 04.09.2021 21:10




Advanced Placement (AP), 04.09.2021 21:10



English, 04.09.2021 21:20

Mathematics, 04.09.2021 21:20

Mathematics, 04.09.2021 21:20



Mathematics, 04.09.2021 21:20